Prevent random downloading and leakage by setting
document access right and history management, preventing
loss and loss due to paper management
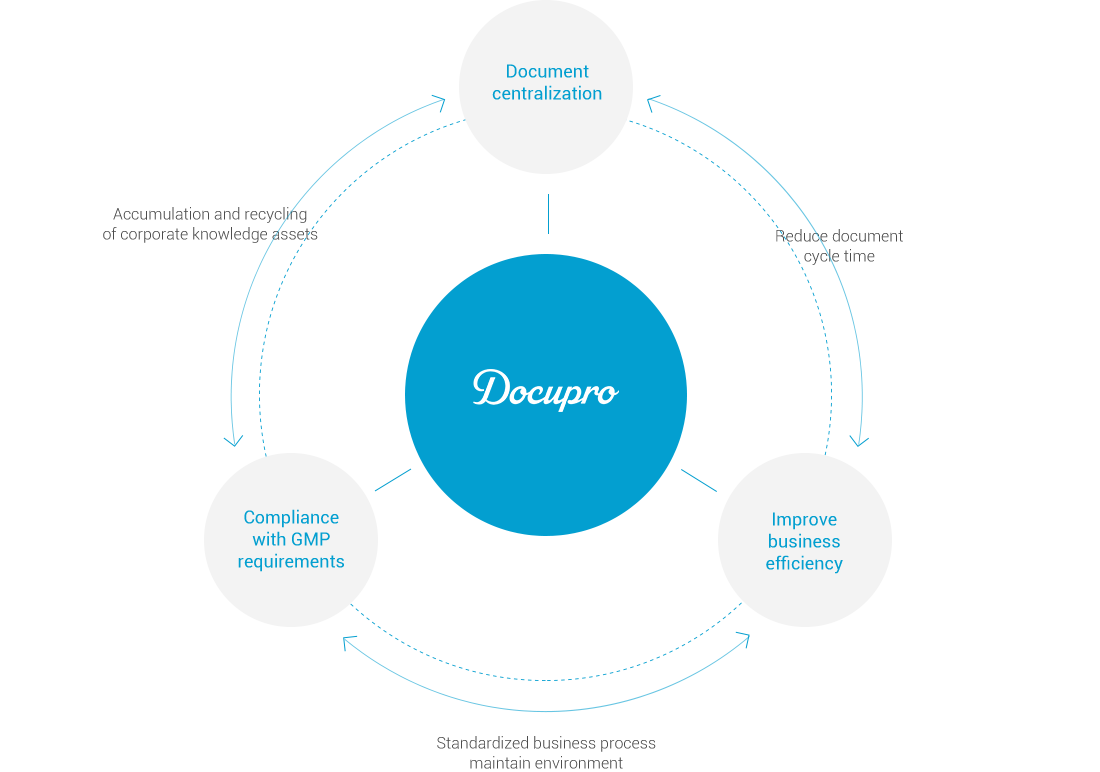
Docupro for GMP
Through SOP process management, compliance with GMP regulations,
It is an electronic document management system for efficient operation of documents.
|
Standard ECM engine based system
|
Docupro is GMP Compliance System
Perform real-time settlement of payment of SOP, validation, and |
|
Convenient integrated search
You can search the text of the document by folder, category, |
Manage content integration
Various metadata types such as document storage, version |
|
Content management according to
international standards
Provides the ability to manage the entire lifecycle of a document, |
Based on proven Alfresco
Developed based on Alfresco, a global open source content |
|
Workflow-based centralized system
Workflow-based electronic approval allows review and approval |
|
|
Benefit
Manufacturing Units Occur per 1Batch
Reduced turnaround time. Maximum yearly 30% reduction in production cost
|
Benefit
Document management occurs during distribution
Automatically records everything. Real time monitoring
|
