Docupro for GMP
Through SOP process management, compliance with GMP regulations,
It is an electronic document management system for efficient operation of documents.
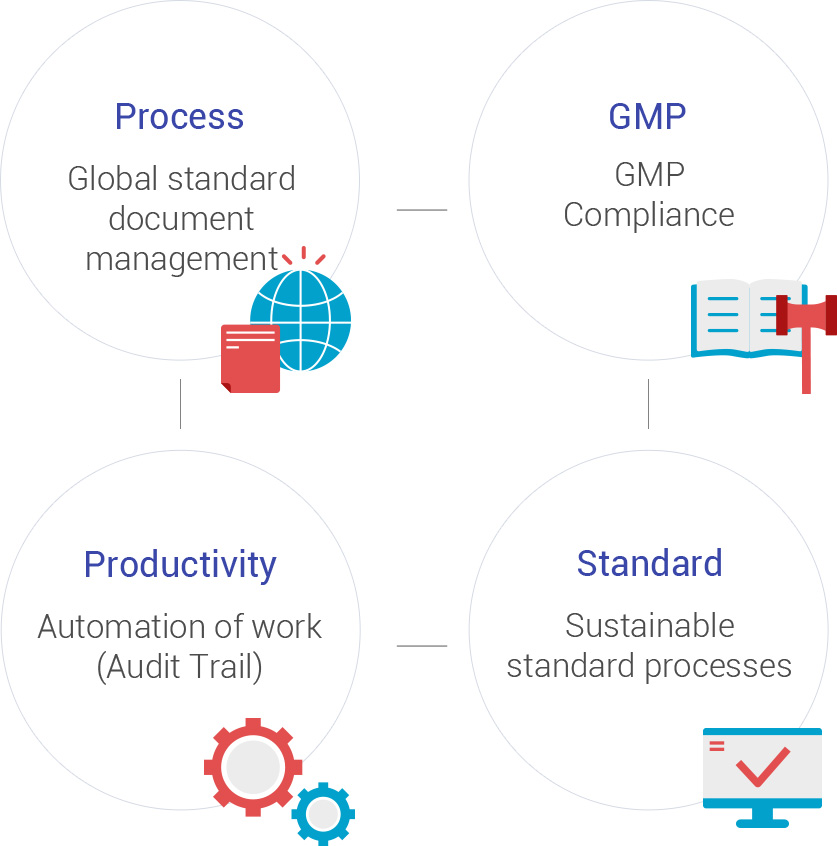

- Compliance with integrated regulatory requirements
- GxP support enables global industry entry, low cost Establish / realize infrastructure system that can meet international regulatory requirements
- Improve management efficiency
- Document LifeCycle Manage and improve quality through processes Enhance document security, Document access right setting and history management, loss against paper document management, prevention of loss, etc.
- Reduce cost
- Process standardization and optimization, cost savings through paperless implementation
- Change in business paradigm
- Sustainable standard process management can speed up your work, prevent retrospective and random modification of documents, and browse without space / time constraints on required documents.