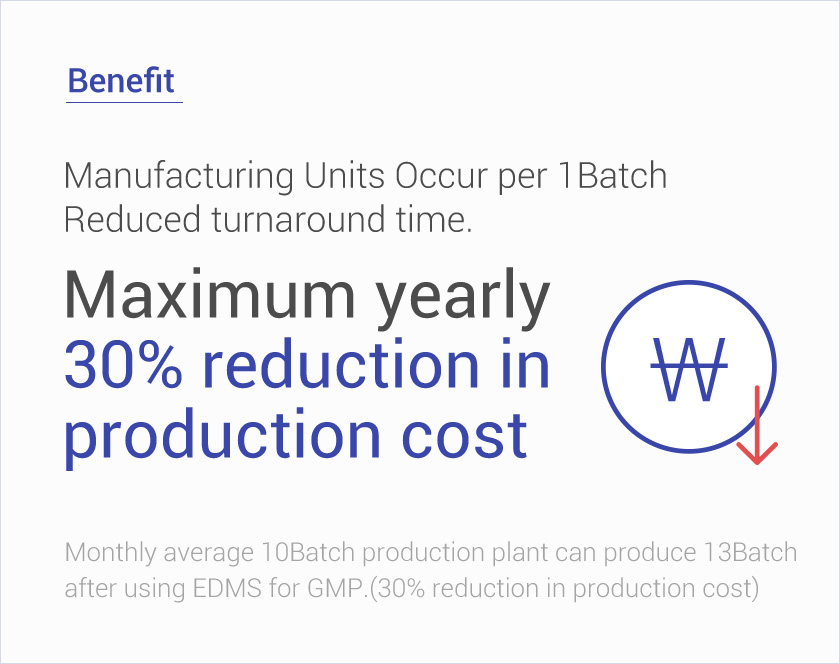
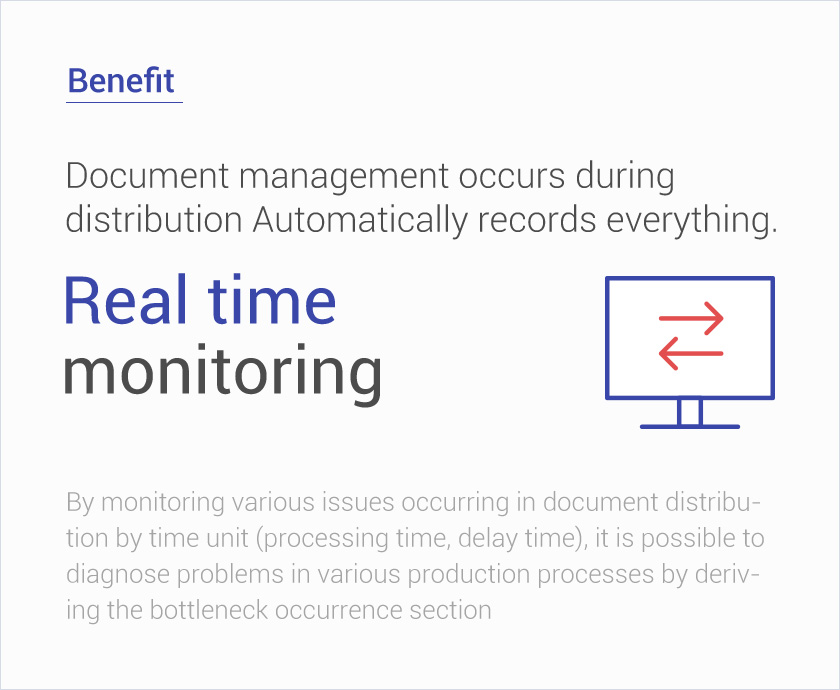
Docupro for GMP
Through SOP process management, compliance with GMP regulations,
It is an electronic document management system for efficient operation of documents.
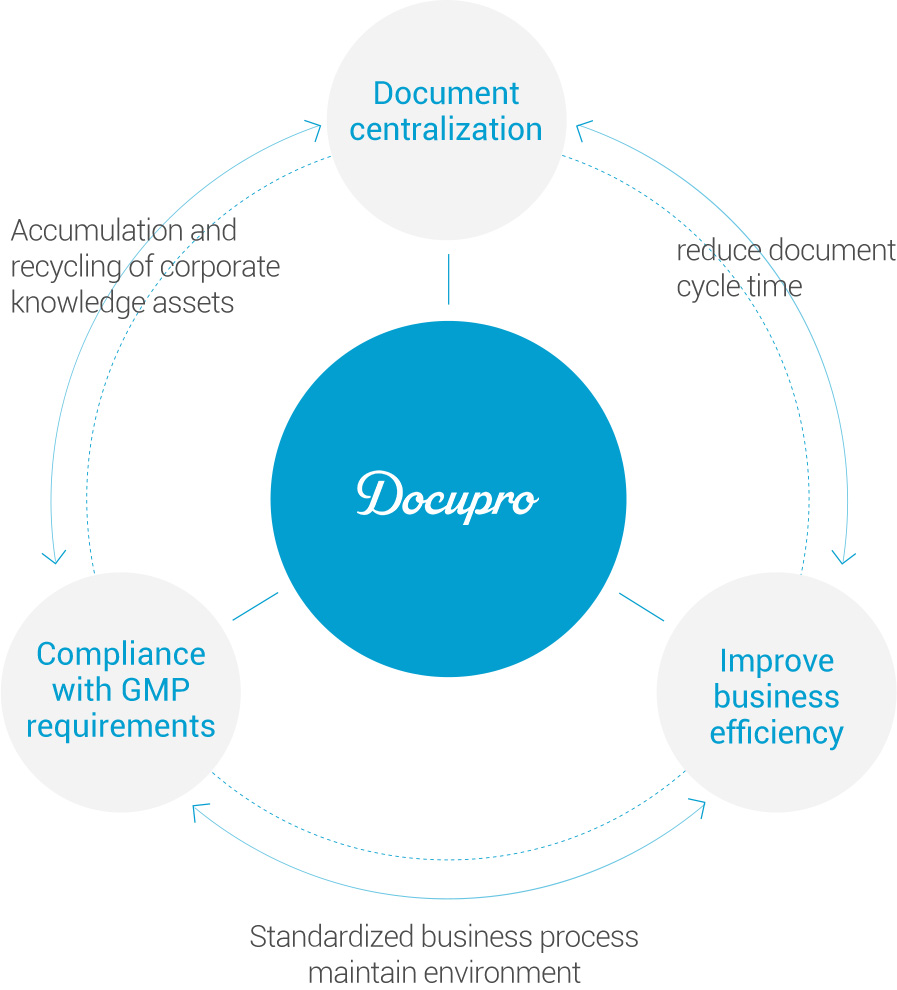
- Standard ECM engine based system
- Prevent random downloading and leakage by setting document access right and history management, preventing loss and loss due to paper management
- Convenient integrated search
- You can search the text of the document by folder, category, and tag by using Wiseolr integrated search engine
- Content management according to international standards
- Provides the ability to manage the entire lifecycle of a document, from creation to retirement. Complies with the CMIS standard specification, which is an international standard.
- Workflow-based centralized system
- Workflow-based electronic approval allows review and approval without time / space restrictions
- Docupro is GMP Compliance System
- Perform real-time settlement of payment of SOP, validation, and manufacturing records, which are documents managed by GMP that changes rapidly, online
- Manage content integration
- Various metadata types such as document storage, version management, and history management can be managed according to various types of policies for each organization, and policy management and relationship management between documents can be performed
- Based on proven Alfresco
- Developed based on Alfresco, a global open source content management system used by more than 3,000 companies in 180 countries worldwide
- GMP(Good Manufacturing Practice) : Global standard for pharmaceutical manufacturing management
- Contents Management Interoperability Service (CMIS) : Service standards that an application can use to manipulate content stored in the repository